Novel isoquinolone PDK1 inhibitors discovered through fragment-based lead discovery.
Johnson, M.C., Hu, Q., Lingardo, L., Ferre, R.A., Greasley, S., Yan, J., Kath, J., Chen, P., Ermolieff, J., Alton, G.(2011) J Comput Aided Mol Des 25: 689-698
- PubMed: 21779981
- DOI: https://doi.org/10.1007/s10822-011-9456-7
- Primary Citation of Related Structures:
3SC1 - PubMed Abstract:
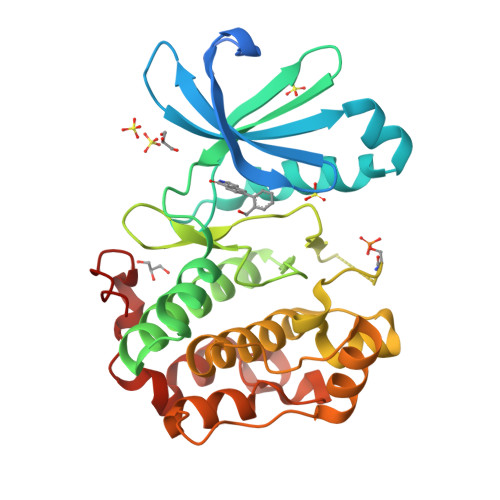
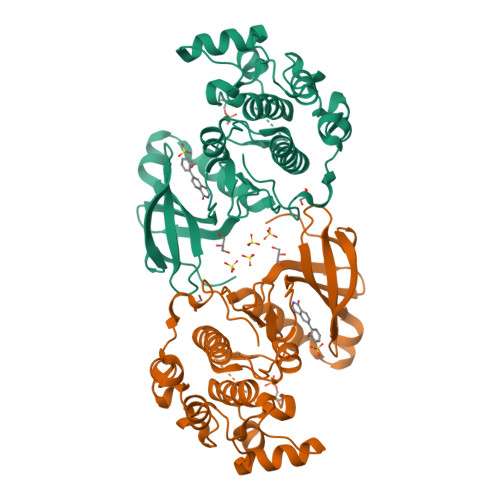
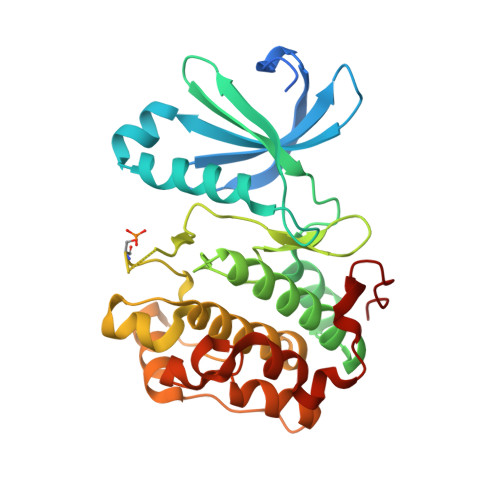
Phosphoinositide-dependent kinase-1 (PDK1) is a critical enzyme in the PI3K/AKT pathway and to the activation of AGC family protein kinases, including S6K, SGK, and PKC. Dysregulation of this pathway plays a key role in cancer cell growth, survival and tumor angiogenesis. As such, inhibitors of PDK1 offer the promise of a new therapeutic modality for cancer treatment. Fragment based drug screening has recently become a viable entry point for hit identification. In this work, NMR spectroscopy fragment screening of PDK1 afforded novel chemotypes as orthogonal starting points from HTS screening hits. Compounds identified as hits by NMR spectroscopy were tested in a biochemical assay, and fragments with activity in both assays were clustered. The Pfizer compound file was mined via substructure and 2D similarity search, and the chemotypes were prioritized by ligand efficiency (LE), SAR mining, chemical attractiveness, and chemical enablement of promising vectors. From this effort, an isoquinolone fragment hit, 5 (IC(50) 870 μM, LE = 0.39), was identified as a novel, ligand efficient inhibitor of PDK1 and a suitable scaffold for further optimization. Initially in the absence of crystallographic data, a fragment growing approach efficiently explored four vectors of the isoquinolone scaffold via parallel synthesis to afford a compound with crystallographic data, 16 (IC(50) 41.4 μM, LE = 0.33). Subsequent lead optimization efforts provided 24 (IC(50) 1.8 μM, LE = 0.42), with greater than fivefold selectivity against other key pathway kinases.
Organizational Affiliation:
Pfizer Global Research and Development, San Diego, CA 92121, USA, mcjohnson@inchemdesign.com